Ch3oh Lewis Structure Molecular Geometry
CH3OH Lewis construction , Molecular Geometry and Shape
Methanol or Methyl alcohol is one of the compounds that are used to understand the molecular geometry, bonds, and much more in Organic chemistry. This chemical compound has a hydroxyl group ( OH) fastened to the methyl grouping, and that is where it gets its proper noun of "methyl alcohol." To make it easier for you lot to understand, assume that 1 hydrogen atom of methane or CH4 is substituted by a hydroxyl group, resulting in Methanol having the chemical formula of CH3OH.
Methyl booze is a light, colorless, and volatile liquid with an alcoholic smell similar to ethanol. The molecule'due south structure is easy to understand, and one tin can also use this example to study more than complex structures in organic chemistry. To understand the structure and shape of this chemical compound, it is vital to know its valence electrons and Lewis structure.
CH3OH Valence Electrons
Methanol consists of one carbon cantlet, 3 Hydrogen atoms, and one hydroxyl group. To know the total number of valence electrons, we have to know the valence electrons of all the atoms individually:
Carbon has iv valence electrons in its outer shell, hence the valence electrons in Carbon= four.
Hydrogen has only i valence electron, but as in that location are three Hydrogen atoms in this compound, the total number of valence electrons for Hydrogen = iii*1= 3.
Oxygen has six valence electrons in its outer shell and needs two electrons to follow the octet rule; hence its valency is 6.
Hydrogen fastened to the Oxygen in the hydroxyl group has one valence electron; hence its valency is i.
Full number of valence electrons in CH3OH = 4 + 3+6+1
= 14
Thus the total number of valence electrons in CH3OH ( Methanol) is fourteen.
Octet Rule
In chemistry, all the atoms tend to become inert by attaining the electronic configuration of the noble gas that has 8 electrons in its outer vanquish. Hence all the atoms tend to form bonds in achieving this configuration and become stable. This dominion has some exceptions in chemistry, but majorly, all elements follow this octet rule.
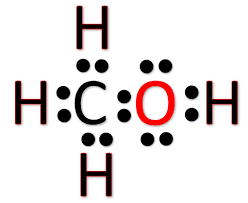
CH3OH Lewis Construction
Lewis dot structure is a pictorial representation of the molecule, it's bonding with other atoms and the organisation of atoms in the compound. It helps in knowing the number of bonded electrons, lone pairs, and the chemical compound'south molecular shape. Valence electrons aid in drawing this Lewis structure, as all the electrons are shown by using dots, and the straight lines represent the bonds formed between the molecules.
Hither in CH3OH,
In that location are a total of 14 valence electrons in the chemical compound. Carbon has a steric number of four equally it has four valence electrons in its outer shell. In Methanol, Carbon is the key atom, and all the other atoms are placed around it.
For drawing the construction, you can place four electrons ( as dots ) around the primal carbon atom in all four directions. Now all the Hydrogen atoms have ane valence electrons, and all these three atoms form a bond with Carbon by sharing one electron of the Carbon atom. To correspond these bonds, depict straight lines between three Hydrogen atoms and the central carbon cantlet.

Hydroxyl grouping ( OH) shares one valence electron with Carbon, and thus this hydroxyl group forms one bail with the Carbon past sharing its valence electron. There are four valence electrons left in the outer shell of the oxygen cantlet as information technology shares one of its half-dozen valence electrons with Hydrogen and another one with the Carbon atom. Notwithstanding, in that location are iv valence electrons on the Oxygen atom that forms two lone pairs of electrons around information technology. Thus all the valence electrons of the Carbon atoms accept now formed bonds, and at that place are no lone pairs or non-bonded electrons on the primal Carbon atom but Oxygen has 2 lonely pairs of electrons.
CH3OH Molecular Geometry
Now that we know the Lewis structure of CH3OH, information technology is easy to depict the compound'due south molecular geometry. While drawing the Lewis construction for CH3OH, yous volition detect that the Carbon atom will have 3 bonds with three hydrogen atoms and one bond with the Hydroxyl Group.
Equally the Carbon has 4 valence electrons that grade the bonds with other atoms, it shows sp3 hybridization.
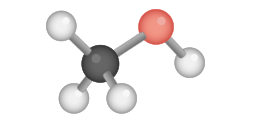
CH3OH Shape
The hybridization of the central cantlet ( Carbon ) in CH3OH is sp3, which means that information technology should form a tetrahedral shape, but it doesn't form this shape exactly. The shape of Methanol is bent because the hydroxyl group ( OH) contains two lone pairs of electrons, which crusade the repulsion between the bonded pair of electrons and the non-bonded pair of electrons in the compound. These repulsion forces atomic number 82 to information of a bent structure.
According to some theories, it is also believed that CH3OH has 2 geometric centers, ane for the Carbon atom and another for the Oxygen atom in the hydroxyl group. The central carbon atoms course four sigma bonds and have no alone pairs, which results in the germination of a tetrahedron. Simultaneously, the Oxygen atom forms 2 sigma bonds and two lone pairs of electrons, which causes a bent in the bond angle due to the repulsion forces. Thus Oxygen has a bent tetrahedral shape, resulting in the aptitude shape of Methanol.
Concluding Remarks
The construction of Methanol or CH3OH is insufficiently easy to study as the valency of the central Carbon cantlet is fully satisfied, and there are no lone pairs on the carbon atom. The cantlet shares three of its four valence electrons with Hydrogen atoms and rests one electron with the hydroxyl grouping. Central Carbon cantlet has sp3 hybridization and a bent molecular shape due to the repulsion between lonely pairs on Oxygen and the bonded pairs in the molecule.
Ch3oh Lewis Structure Molecular Geometry,
Source: https://geometryofmolecules.com/ch3oh-lewis-structure-molecular-geometry-and-shape/
Posted by: robinsonbitterephe56.blogspot.com


0 Response to "Ch3oh Lewis Structure Molecular Geometry"
Post a Comment